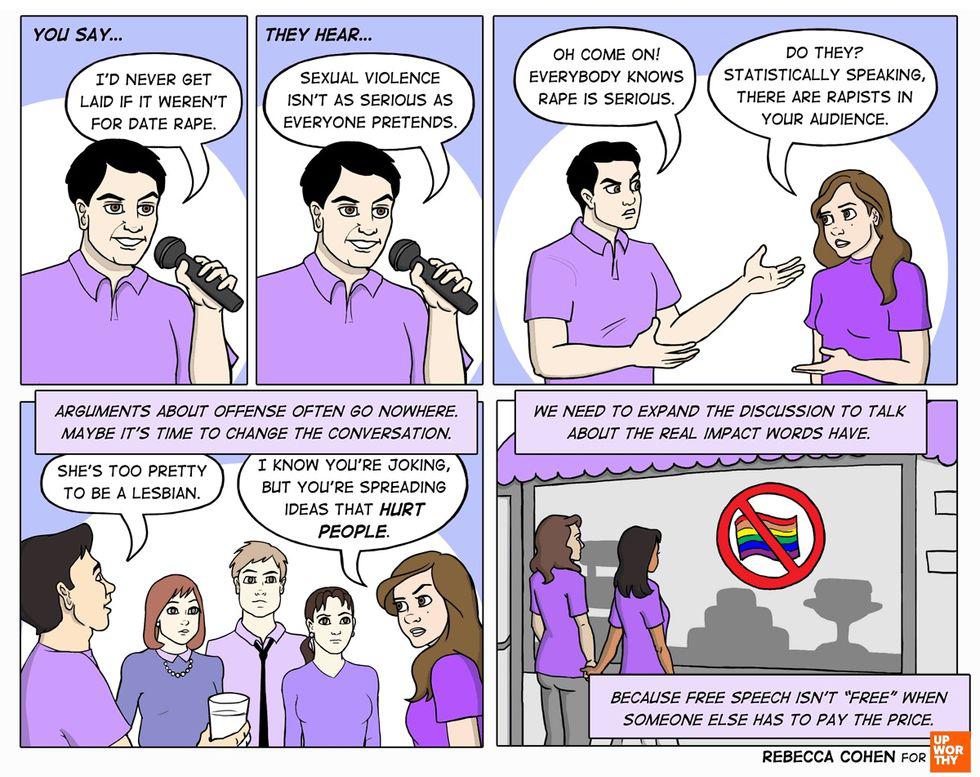
Simply put, strep throat is miserable.
Streptococcal pharyngitis (the dreaded condition’s full name) is a bacterial infection that causes a painful sore throat and a fever. And for the unlucky people who get it, symptoms can also include headaches, belly pain, and a rash.
At least there’s Netflix. Photo by iStock.
Strep throat and infections like it are much more than a schoolyard nuisance.
Strep is just one of the group A streptococcal (GAS) infections. That’s science for the dream team of gnarly illnesses that also includes pneumonia and flesh-eating infections.
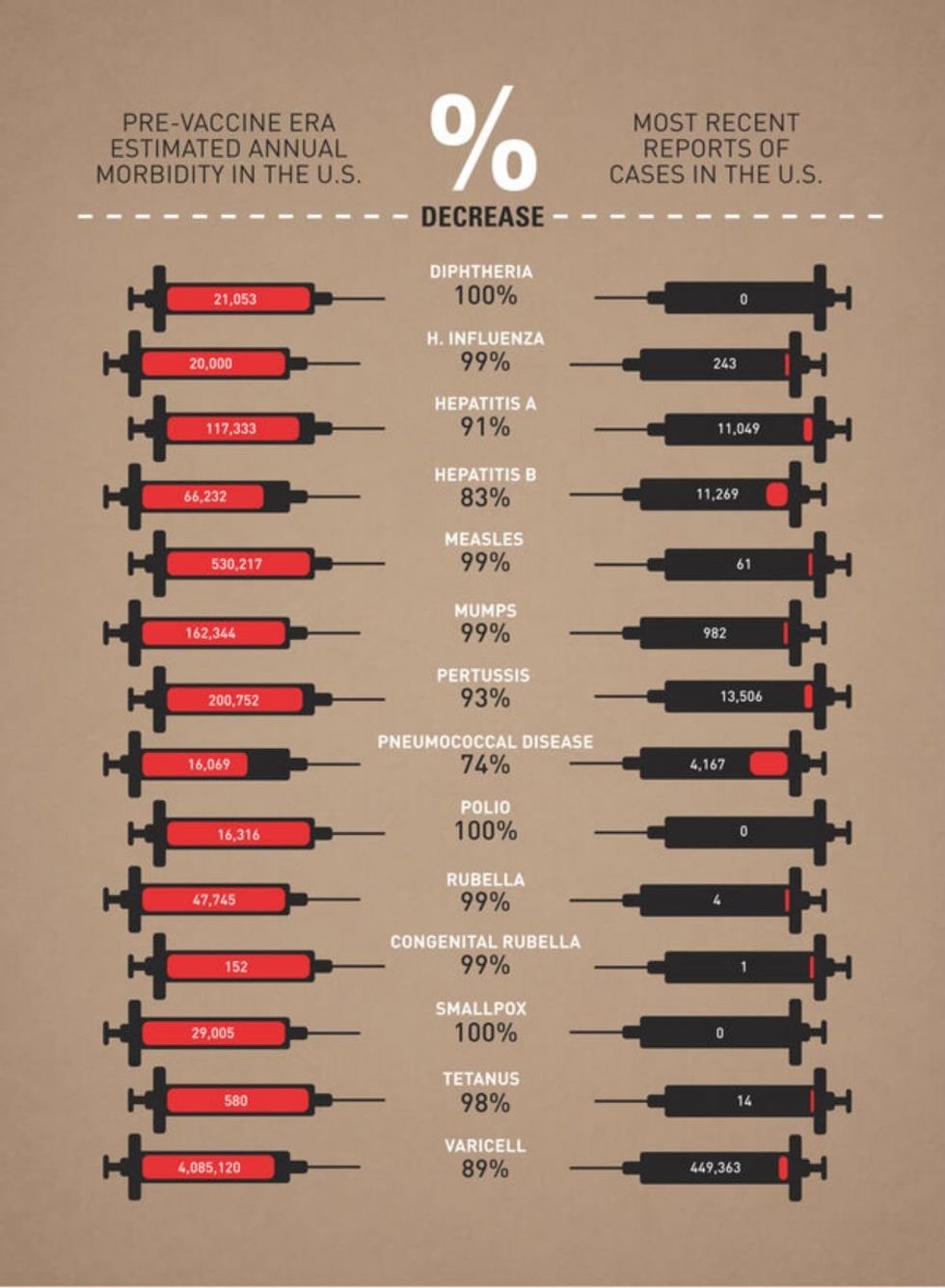
Each year, strep throat affects 7.3 million people in the U.S. and streptococcal diseases result in 1,200 to 1,600 deaths.
But we’re one step closer to eliminating strep throat completely, thanks to a new vaccine.
StreptAnova, a vaccine developed by Memphis-based physicians James Dale and Gene Stollerman, was designed to prevent GAS infections, particularly in children and teens, who are most susceptible to the illnesses.
So long, strep throat. Photo by iStock.
The vaccine is especially important for people in developing countries. In an interview with Upworthy, Dale explained that strep throat infections can lead to rheumatic fever and rheumatic heart disease. Those diseases cause 233,000 deaths each year.
“A vaccine designed to prevent rheumatic fever and invasive infections could have a major impact on the health of millions worldwide,” he said.
Before the vaccine is made available to the public, it must be tested, tested again, and tested some more.
Before the U.S. Food and Drug Administration approves a vaccine, it must undergo a battery of trials, typically conducted in three phases. Phase 1 trials are conducted with a very small group of closely monitored individuals.
For StreptAnova, the Phase 1 trial is taking place at the Canadian Center for Vaccinology in Halifax, Nova Scotia. There, 45 healthy adults will receive three injections of StreptAnova over six months. Physicians will follow up a year later to assess the individuals and their response to the drug.
Science-ing the hell out of some samples. Photo by iStock.
Phase 2 and Phase 3 trials are similar but larger-scale, enrolling a few hundred and a few thousand people, respectively. The larger trials provide proof of effectiveness.
Following the tests, there’s a lot of paperwork, applications, licenses, and procedures. If the vaccine passes muster, it’s made available to the general public.
A vaccine usually requires 10 to 15 years of research and testing before it shows up at the doctor’s office. But Dale is willing to wait.
“I do not really have a ‘Plan B,’” he said. “I plan to continue working on the clinical development of GAS vaccines until they are available to everyone that could benefit.”
Eye protection on, ladies. We’ve got science to do. Photo by iStock.
While preventing strep throat completely is several years away, there are plenty of ways to stay healthy.
Strep is easily contagious, as any contact with droplets from an infected person’s cough or sneeze can be all it takes to get yourself sick.
Sorry, you have strep. Don’t go to work. And stop sharing sodas. Photo by iStock.
If you’re living or working with someone with strep throat, keep your hands clean and try to avoid touching your eyes and mouth. And for once in your life, don’t share. It’s best to avoid sharing personal items like cups or utensils with anyone who may have symptoms.
Wash your hands long enough to sing “Happy Birthday” twice. Photo by iStock.
But don’t worry, 24 hours after the infected person begins antibiotics, the risk of contracting the illness decreases dramatically, and you can go back to preparing for flu season, which is just around the corner.