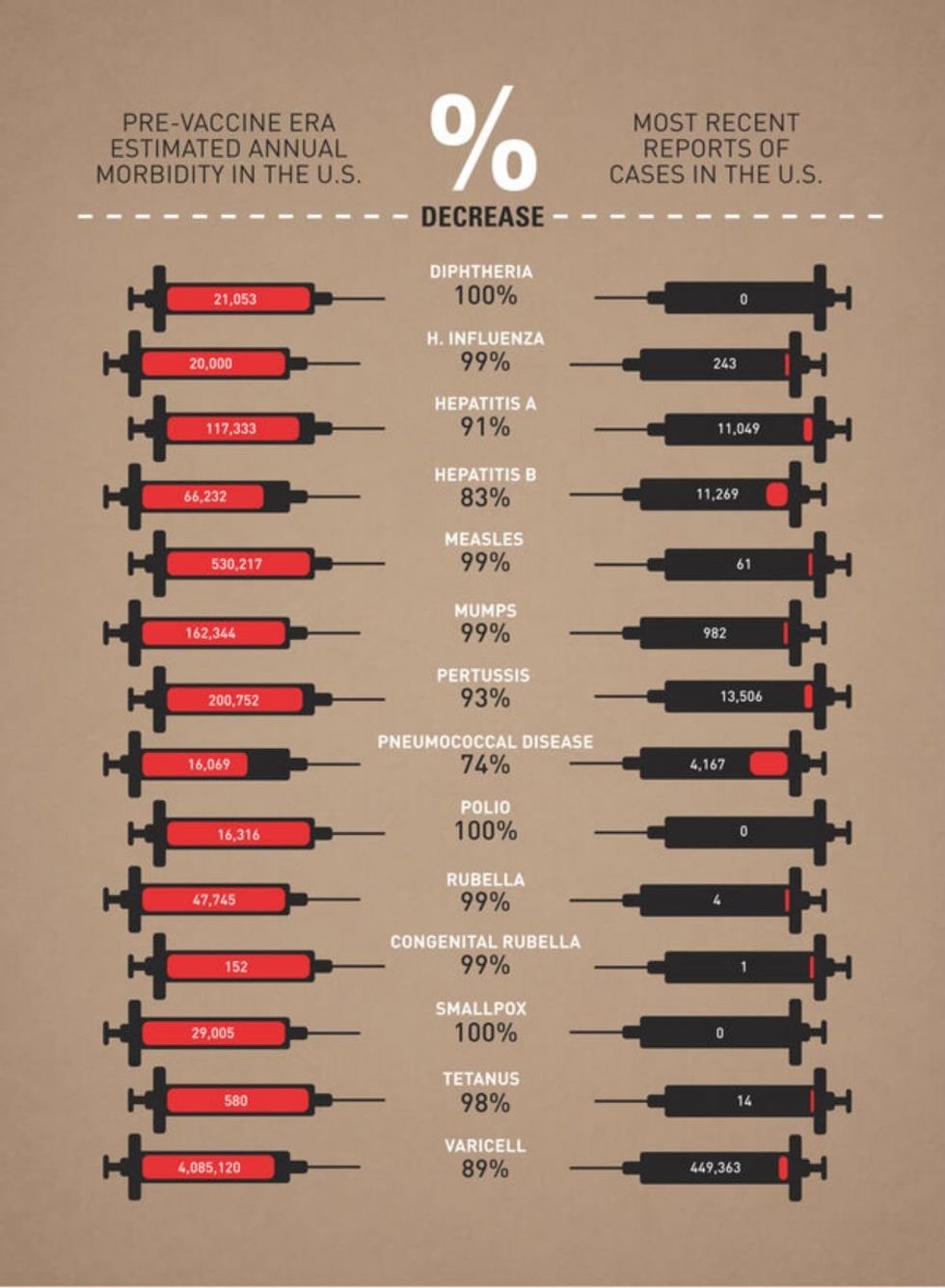
Kim Kardashian, former breaker of the Internet and star of “That Show You Watch on the Treadmill,” is pregnant.
But will she get her baby body back??? AMERICA NEEDS TO KNOW!!!
Unfortunately, pregnancy hasn’t been all baby bump, push present, and smooth sailing for the reality star. Like most expectant mothers, Kardashian has suffered from occasional, painful bouts of morning sickness.
Fortunately, unlike most of her pregnant cohort, Kardashian was able to take that suffering, grab it by the collar, and monetize the ever-loving bejeezus out of it.
For those who hate squinting, that’s Kardashian singing the praises of a morning sickness drug called Diclegis.
Just, you know, giving it some completely spontaneous love on social media. Like normal people do. For their 37 million friends. A genuine, heartfelt, totally-not-secretly-highly-compensated-stealth-endorsement from your bud, who definitely has real actual personal experience using the product in question.
Who wouldn’t believe her?
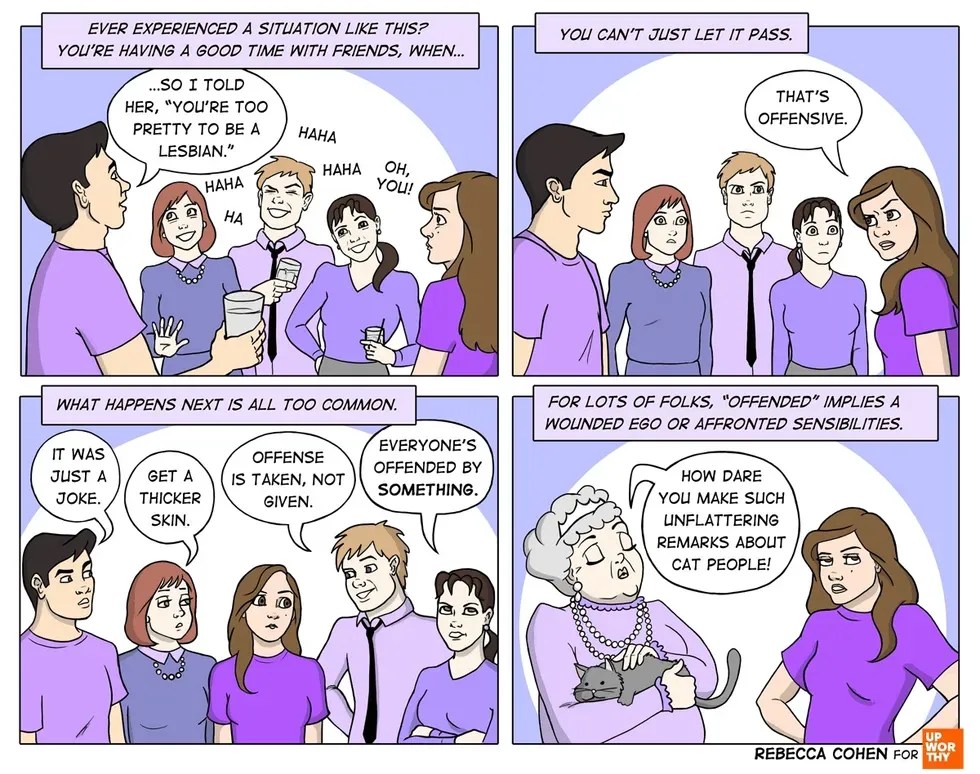
The U.S. Food and Drug Administration, for one.
An FDA official, seen here making sure some lettuce isn’t a deadly biohazard. Photo by the FDA.
Turns out, Kardashian’s post was, in fact, a paid advertisement. (I know. Try to remain calm.) Not only was it a paid advertisement, but it was a paid advertisement that left out some pretty crucial information about some of the drug’s pretty nasty potential side effects.
Because of the vast reach of Kardashian’s Instagram feed, the agency was compelled to issue a response — and it was an ornery one:
“The social media post is false or misleading in that it presents efficacy claims for DICLEGIS, but fails to communicate any risk information associated with its use and it omits material facts. Thus, the social media post misbrands DICLEGIS within the meaning of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and makes its distribution violative. … These violations are concerning from a public health perspective because they suggest that DICLEGIS is safer than has been demonstrated.”
Two things should be immediately clear from the FDA statement.
1. It would make amazing Adele lyrics…
“Amazing.”
And…
2. Taking medical advice from a reality TV star is a terrible idea.
Unfortunately, Kim Kardashian is just the tip of the iceberg…
Prescription drug ads in general — even ones that do get their facts right — are kind of the worst.
Abilify has been great for my anxiet … AAAGGHH A SENTIENT BATHROBE IS FOLLOWING ME AAAAGGHH! Photo by Raza Syed, used with permission.
If you watch any TV at all, chances are you’ve seen at least a bajillion-and-a-half commercials urging you to “talk to your doctor” about Nexium, Cialis, Novorex, Fontainebleau, Prospector Pete’s Tooth Caulk, or what have you if you experience any number of vague, over-broad symptoms.
Sneezing? Talk to your doctor about oxycodone. Photo via iStock.
And sure, you could demand your doctor write you this or that prescription. And they’d probably smile and nod. Most likely, they’d try to gently talk you down. But there’s a chance they would just decide it’s not worth the trouble and give you what you want — even if it’s a drug you don’t actually need. And that’s where the problem lies.
Asking a professional to disregard their hard-won expertise on the advice of a commercial is kind of ridiculous when you think about it.
How ridiculous? Just … imagine any of the following scenarios:
“Donating to earthquake relief in Haiti? Talk to your charity about sending your cash to Sweden instead.”
“Claiming a deduction for that charitable gift you just made? Talk to your accountant about turning it into a home mortgage interest deduction!”
“And speaking of home, why does your home have to be made of bricks and mortar? Talk to your builder about graham crackers and strawberry cream cheese.”
Sturdy as a rock! Photo by rcstanley/Flickr.
That’s what drug ads are trying to get you to do.
Doctors aren’t perfect, of course. They’re human, and they do get things wrong from time to time. But drug commercials are essentially hoping you’ll go to your physician and say, “Sure, you’ve got professional integrity, six years of graduate school, and took an oath to do no harm, but what about this 30-second B-roll of women in bathtubs voiced by the third lead actor from ‘Suits’ that I just saw? How does that stack up?”
They’re trying to Kardashian you.
Not only are these ads often misleading, they also help drive up the price of drugs.
It’s no secret that Americans like things bigger and better.
A “small” in America.
Our towels are plusher. Our French fries are more fried. Our hats are larger.
And our drugs are the most expensive in the world — often double the price of what they are in other similarly wealthy countries.
The United States is one of only two countries in the world that allow direct-to-consumer drug ads — and we pay extra for medicine because of it.
New Zealand is the other. Obviously, there’s worse company we could be in. Photo by thinboyfatter/Flickr.
Turn on British TV, and you won’t see Benedict Cumberbatch striding purposely across a cricket pitch, saying, “Pardon me, sir, but if it’s not terribly much of a bother, would you mind inquiring with your physician about Crumpetrex?” Watch television in Germany, and no one will urge you to “ASKEN ZE DOCTOR FIR SCHNITZELGRAZ!”
We are the only suckers who get those ads shoved in our faces. (New Zealanders do too, but they get hobbits in exchange.)
And pharmaceutical companies go buck-wild on these ads … spending $20 billion over the past five years.
That cost gets passed on to sick people (and to people who pester their dermatologist until she throws up her hands and signs over a prescription for Cialis so she can move on to her next wart-freezing already).
Regardless of our reasons for needing a medication, that’s not a cost we should be paying.
Like Kim Kardashian’s Instagram feed, drug commercials serve pretty much no practical purpose — and there’s no reason they couldn’t quietly disappear forever.
Serious doctors mean business. Photo via iStock.
That’s what the American Medical Association — the largest professional organization of physicians in the United States — wants, which is why it voted to call for a ban on the ads last week.
“Today’s vote in support of an advertising ban reflects concerns among physicians about the negative impact of commercially driven promotions and the role that marketing costs play in fueling escalating drug prices,” Dr. Patrice Harris, a member of the AMA board, told the Chicago Tribune. (Come on, Adele, are you seeing this? So much gold here!)
That’s a great first step. But there’s still more that could be done.
Unfortunately, the AMA vote doesn’t address subtle social media ads like Kardashian’s, which could lead pharmaceutical companies to simply shift their ad dollars toward efforts on platforms like Facebook and Instagram. (And let’s be honest, who among us wouldn’t share a Facebook-optimized George Takei meme featuring dancing arthritic cats, sponsored by Celebrex?)
Meow to your doc … purrrrr about Celebrex! Photo via iStock.
It also doesn’t address direct-to-physician marketing, in which drug companies use sales representatives to tout the benefits of their medications to doctors in person, which accounts for a huge portion of pharmaceutical industry spending — in addition to just being generally kind of shady. (“Funny, my doctor prescribed me the exact same medication that was featured on that nifty pen in his office!)
If we really want our drugs to pull even price-wise with the Canadas and Norways of the world, a federal law that calls for transparency, competition, and fair pricing needs to be passed and enforced.
As for Kim Kardashian? She ultimately made good on her endorsement deal…
…and satisfied the FDA in the process.
Maybe not … quite as catchy. But … progress?